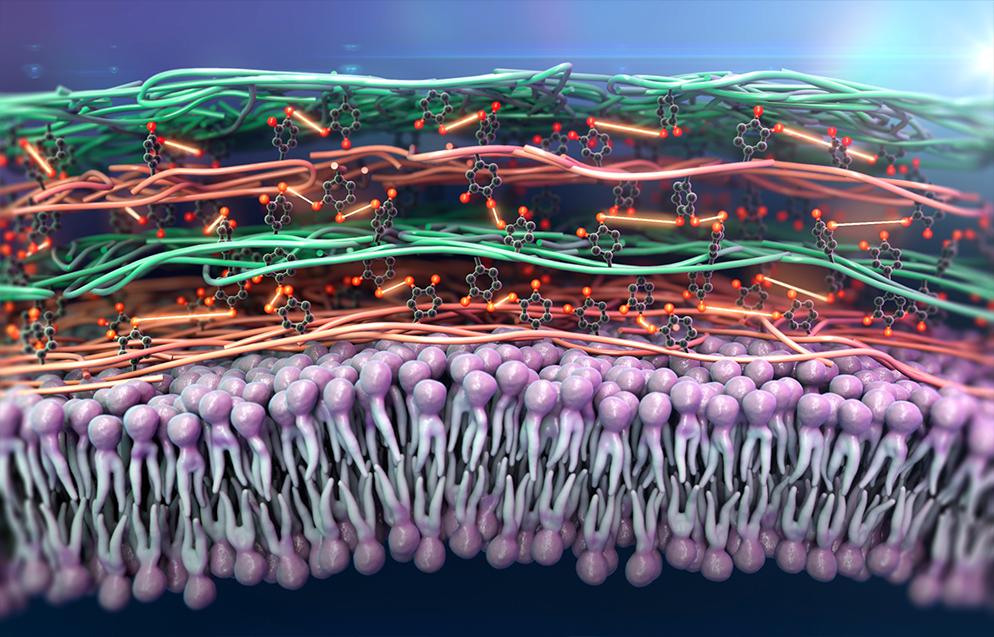
Enzyme-based crosslinking technologies for hydrogel nanofilm fabrication
Potential application of hydrogel nanofilm technology in islet transplantation for cytoprotection
Professor Nathaniel Hwang in the School of Chemical and Biological Engineering has been using enzyme-based crosslinking technologies for biomedical applications. His recent work has led to the development of a "cell caging" technology that could potentially be used to treat type 1 diabetic patients without the need for immunosuppressants.
Type 1 diabetes is an autoimmune disease that results from the destruction of pancreatic beta cells, which causes insulin deficiency and hyperglycemia1. The disease is caused by the development of Th1 cells that react to autoantigens in pancreatic beta cells, leading to Type 1 immune responses and inflammation that can destroy the pancreatic tissue. As a result, type 1 diabetes patients require a continuous source of insulin replacement since their beta cells, which produce insulin, are removed by the immune system.
For many years, the main approach to treating type 1 diabetes involved administering insulin injections. But in 2000, allogeneic pancreatic transplantation became an alternative option, which has since been carried out on over 6,000 patients in the US2. Nonetheless, there is still a risk of rejection by the immune system with this approach. Immune rejection can occur due to ABO antigen mismatch or HLA antigen mismatch, which means using immunosuppressants is unavoidable.
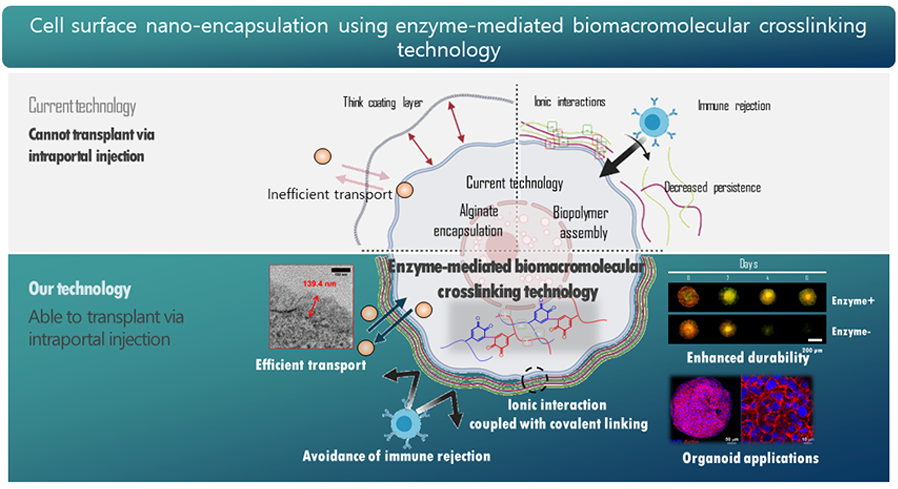
Over the past 20 years, researchers have been working on various methods to induce immune tolerance, but there are limitations to achieving complete immune suppression through immune tolerance alone. Immune tolerance-based immune suppression has shown little effectiveness in preclinical studies on large animals. However, studies have been reported proving the effectiveness of immune evasion strategies that reduce immune system exposure. In particular, the encapsulation of the donor's pancreas has been developed as an innovative pancreas transplantation material to physically separate it from the recipient's immune system to reduce antigen exposure.
The pancreas encapsulation method uses various biomaterials to allow for more stable pancreas transplantation, as immune cells cannot recognize the transplanted pancreas. However, there is currently no clinically applicable pancreas encapsulation technology available due to the inability to adjust the thickness of the biomaterial capsule to match the actual pancreas, and the gradual removal of the biomaterials used in encapsulation by external environmental stress, making continuous blood sugar control impossible.
Prof. Hwang’s group fabricated a new concept of the pancreatic β cell spheroid caging system by an enzymatic cross-linking-based hydrogel nanofilm with high stability and cytoprotective property3. The system utilizes a hydrogel nanofilm composed of biocompatible polysaccharide layers of glycol chitosan (GC) and hyaluronic acid (HA) cross-linked by Streptomyces avermitilis-derived tyrosinase (SA-Ty)-mediated enzymatic reaction. The SA-Ty has shown superior reactivity on long-chain polymers, which accelerates polymer cross-linking. The hydrogel nanofilm has demonstrated high stability and cytoprotective property. It also acts as a physical barrier against the external environment, conserving the hydrogel nanofilm over a long period, as well as enduring external pressure and reducing cell-cell interaction with natural killer cells [Figure 1].
Moreover, the hydrogel nanofilm formed by SA-Ty on mouse pancreatic β cell spheroids has shown the potential to return type 1 diabetes (T1D)-induced mice to normoglycemia. The nanofilm-caged spheroids were confirmed to be successful in engraftment and therapeutic efficacy by analyzing long-term blood glucose concentration, intraperitoneal glucose tolerance test (IPGTT), and immunohistochemistry.
This hydrogel nanofilm technology can be applied to both single cells and cell organoids and is expected to be applicable to xenogenic organ transplantation. In other words, the 'cell cage' technology developed by Prof. Hwang's group could potentially overcome the problem of immune rejection, which is the biggest challenge in pancreatic β cell. The goal is to accelerate the clinical development and commercialization of this technology.
References
1. Katsarou, A., et al., Type 1 diabetes mellitus. Nat Rev Dis Primers, 2017. 3: p. 17016.
2. Shapiro, A.M., et al., Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med, 2000. 343(4): p. 230-8.
3. Kim, M., et al., Novel enzymatic cross-linking-based hydrogel nanofilm caging system on pancreatic beta cell spheroid for long-term blood glucose regulation. Sci Adv, 2021. 7(26).
1. Katsarou, A., et al., Type 1 diabetes mellitus. Nat Rev Dis Primers, 2017. 3: p. 17016.
2. Shapiro, A.M., et al., Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med, 2000. 343(4): p. 230-8.
3. Kim, M., et al., Novel enzymatic cross-linking-based hydrogel nanofilm caging system on pancreatic beta cell spheroid for long-term blood glucose regulation. Sci Adv, 2021. 7(26).


