
Developed SUPRA CAR system to improve the safety and efficacy of current cellular cancer immunotherapy.
Developed a platform for engineering advanced logic and cell–cell interactions in human immune cells.
Professor Jang Hwan Cho in the School of Chemical and Biological Engineering has been developing novel chimeric antigen receptors (CARs). CAR-expressing T cell transfer to patients is a promising approach for cancer immunotherapy, as indicated by several studies1. the current development of CAR T cell therapy is, however, still hindered by major obstacles in terms of safety and efficacy2. A better system is urgently needed to enhance the overall effectiveness and safety of CAR T cell therapy, allowing for precise tuning of T cell activation, improved tumor specificity, and independent control of various signaling pathways and cell types.
The inflexible design of CAR T cells utilized in clinical studies presents a challenge for modification without reengineering the T cells. The current composition of CAR designs is fixed, featuring a singular antigen-specific single-chain variable fragment (scFv) and intracellular signaling domains (CD3ζ and costimulatory domains). When the constant antigen-specific CAR binds to the target antigen, these invariable signaling domains activate simultaneously at a predetermined level. Due to the lack of control over the activation level of CAR T cells, managing toxicities associated with these cells has proven difficult3.
Not only does the current fixed design limit the controllability of CAR T cell activity, but it also restricts antigen specificity and affinity. To ensure high antigen specificity, CAR designs frequently incorporate high-affinity scFvs. However, CARs made with high-affinity scFvs cannot discriminate between antigen densities, which can cause dangerous reactivity against healthy organs expressing low levels of antigens4. Using scFvs with lower antigen affinity, on the other hand, improves antigen density discrimination while potentially compromising antigen specificity. Consequently, the modulation of CAR components other than scFv affinity may be necessary to enhance CAR T cell specificity.
Prof. Cho developed a split, universal, and programmable (SUPRA) CAR system that is designed to enhance the specificity, safety, and programmability of CARs5. The SUPRA CAR system consists of a universal receptor expressed on T cells and a tumor-targeting scFv adaptor molecule. The activity of SUPRA CARs can be finely regulated via multiple mechanisms to limit overactivation, and they can also logically respond to multiple antigens for improving tumor specificity. In his research, Prof. Cho demonstrated that the SUPRA CAR system is a sophisticated system with inducible and logical control capabilities that can improve the safety and efficacy of current cellular cancer immunotherapy.
To broaden the scope of computational possibilities with CARs in various types of immune cells, prof. Cho developed the SUPRA system in seven different innate and adaptive immune cell types. He also identified an inhibitory domain that is functional within the SUPRA CAR system, enabling NOT logic6. Leveraging these advanced tools, Prof. Cho constructed a synthetic immune cell consortium and controlled the polarization of macrophages in an inducible manner. Additionally, he distributed computing between multiple cells, utilizing regulatory T-cell-mediated suppression of conventional CD4+ T cells. Prof. Cho also established a direct cell-to-cell communication channel using a zipFv secretion system and an intercellular AND logic circuit. These extensive applications showcase the versatility of the SUPRA CAR system as a platform for designing complex logic and cell-to-cell interactions in human immune cells. The flexibility of the SUPRA CAR system could provide a foundation for engineering synthetic immune cell consortia to address a variety of diseases, including cancer and beyond.
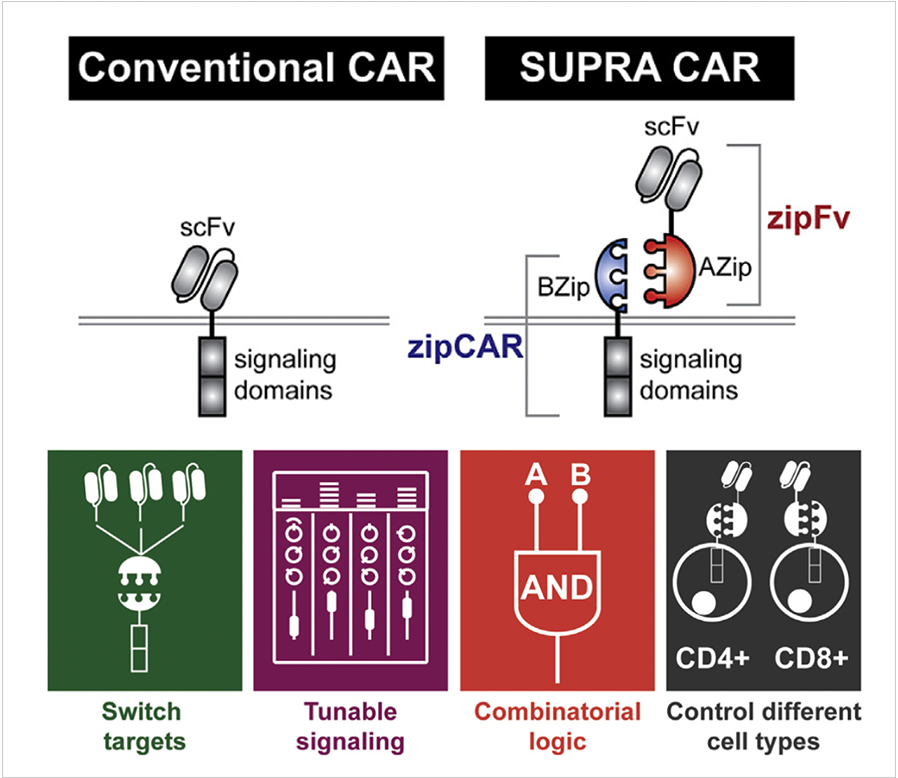
Figure SUPRA CAR design and capabilities5
References
1. A. Ahmad, S. Uddin, M. Steinhoff, CAR-T Cell Therapies: An Overview of Clinical Studies Supporting Their Approved Use against Acute Lymphoblastic Leukemia and Large B-Cell Lymphomas. Int. J. Mol. Sci. 21 (2020), doi:10.3390/ijms21113906.
2. R. C. Sterner, R. M. Sterner, CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021)
3. J. N. Brudno, J. N. Kochenderfer, Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 34, 45–55 (2019).
4. C. L. Bonifant, H. J. Jackson, R. J. Brentjens, K. J. Curran, Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics. 3, 16011 (2016).
5. J. H. Cho, J. J. Collins, W. W. Wong, Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell. 173, 1426-1438.e11 (2018).
6. J. H. Cho, A. Okuma, K. Sofjan, S. Lee, J. J. Collins, W. W. Wong, Engineering advanced logic and distributed computing in human CAR immune cells. Nat. Commun. 12, 792 (2021).
1. A. Ahmad, S. Uddin, M. Steinhoff, CAR-T Cell Therapies: An Overview of Clinical Studies Supporting Their Approved Use against Acute Lymphoblastic Leukemia and Large B-Cell Lymphomas. Int. J. Mol. Sci. 21 (2020), doi:10.3390/ijms21113906.
2. R. C. Sterner, R. M. Sterner, CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021)
3. J. N. Brudno, J. N. Kochenderfer, Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 34, 45–55 (2019).
4. C. L. Bonifant, H. J. Jackson, R. J. Brentjens, K. J. Curran, Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics. 3, 16011 (2016).
5. J. H. Cho, J. J. Collins, W. W. Wong, Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell. 173, 1426-1438.e11 (2018).
6. J. H. Cho, A. Okuma, K. Sofjan, S. Lee, J. J. Collins, W. W. Wong, Engineering advanced logic and distributed computing in human CAR immune cells. Nat. Commun. 12, 792 (2021).


