
Tumor-targeted delivery of immune checkpoint blockades (ICBs) using biomaterials
Gene delivery for in vivo generation of CAR (chimeric antigen receptor) immune cells
Biomaterials-based therapeutic vaccines
Professor Byung-Soo Kim in the School of Chemical and Biological Engineering is developing biomaterials-based immunotherapies for various diseases, including cancer, osteoarthritis, myocardial infarction, and Alzheimer’s disease.
Cancer is the leading cause of death. Up to the present, Immune checkpoint blockade (ICB) is the most effective anti-cancer drug for solid cancers, but there is huge room for improvement. Only 20~40% of cancer patients respond to ICBs, and ICB therapy frequently brings immune-related adverse events. To overcome these limitations, Prof. Kim has researched the delivering of anti-CTLA-4, an ICB, to tumor-specific T cells, promoting the ICB’s efficacy and preventing immune-related adverse events. The delivery has been enabled by carriers made of dendritic cell-derived nanovesicles that present tumor antigen peptides1.
CAR T cell therapy and CAR NK cell therapy have so far shown the most promising clinical efficacy for treating liquid cancers such as B cell lymphoma. However, these cell therapy shows limited clinical efficacy for treating solid cancers. Moreover, ex vivo cell manufacturing is complicated, time-consuming, and cost-ineffective. To address these issues, Prof. Kim has developed a CAR gene delivery technology for in vivo generation of CAR-macrophages. This technology can overcome the limitations of CAR T cell therapy and CAR NK cell therapy for the treatment of solid cancers, effectively eradicating solid tumor growth as well as providing a simple manufacturing process and off-the-shelf, cost-effective therapy2.
Through his research, Prof. Kim has opened up a potential immunomodulatory therapeutic strategy for osteoarthritis. Increasing evidence implicates low-grade inflammation as an important mediator of osteoarthritis pathogenesis. Autoantibodies against type II collagen are found in approximately half of the patients with osteoarthritis. Regulatory T (Treg) cells can mediate immune tolerance and suppress inflammation and, as such, could be an ideal cell for osteoarthritis therapy. To overcome the costs and complications of autologous Treg cell therapy, and the limitations of non-specific immunomodulation, Prof. Kim has developed a therapeutic vaccine for modulating Treg cells in an antigen-specific manner for osteoarthritis treatment. Intradermal injection of lipid nanoparticles loaded with type II collagen peptide and rapamycin into osteoarthritis model animals effectively induces type II collagen-specific anti-inflammatory Treg cells. Therefore, it significantly inhibits joints inflammation and cartilage degradation and relieves pain3. This therapeutic vaccine might be applied to patients with osteoarthritis diagnosed with autoantibodies against type II collagen. This platform technology can also be applied to treat other types of inflammatory diseases.
Alzheimer's disease, the most common cause of dementia, is a complex condition characterized by multiple pathophysiological mechanisms, including amyloid-β (Aβ) plaque accumulation and neuroinflammation in the brain. Current immunotherapy approaches, such as anti-Aβ monoclonal antibody therapy, Aβ vaccines, and adoptive Treg cell transfer, target a single pathophysiological mechanism, which may lead to unsatisfactory therapeutic efficacy. Furthermore, Aβ vaccines often induce T helper 1 (Th1) cell-mediated inflammatory responses. Prof. Kim has developed a therapeutic vaccine composed of lipid nanoparticles loaded with Aβ peptides and rapamycin, which targets multiple pathophysiological mechanisms, exhibits the combined effects of anti-Aβ antibody therapy and adoptive Aβ-specific Treg cell transfer. It can overcome the limitations of current immunotherapy approaches for Alzheimer's disease. The vaccine can effectively produce both anti-Aβ antibodies and Aβ-specific Treg cells, remove Aβ plaques in the brain, alleviate neuroinflammation, prevent Th1 cell-mediated excessive immune responses, and inhibit cognitive impairment in mice. The vaccine is easy-to-prepare and cost-effective, unlike anti-Aβ monoclonal antibody therapy and adoptive Treg cell transfer, which require complicated and costly manufacturing processes4.
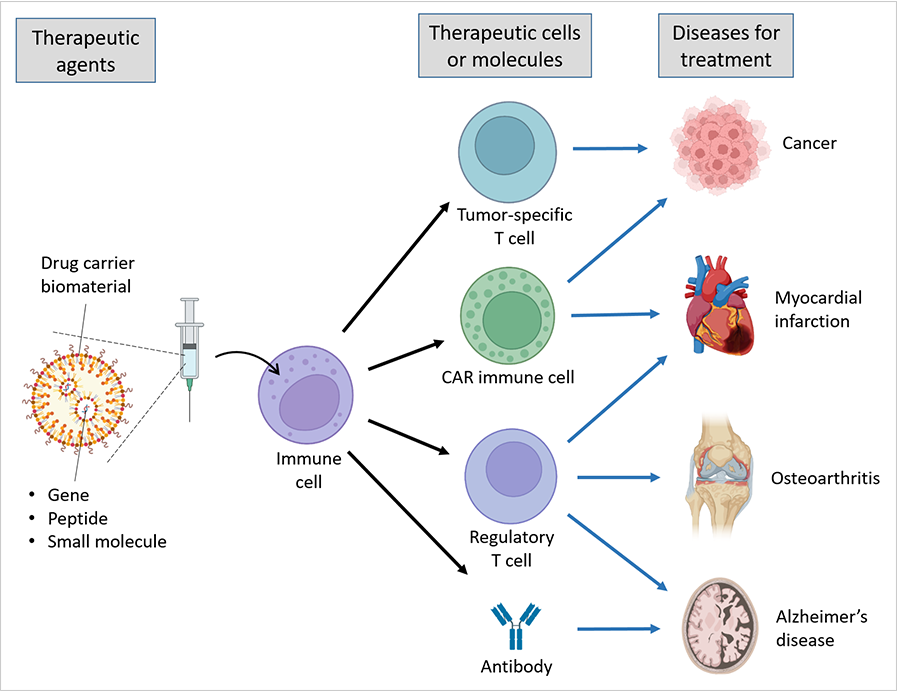
Figure Biomaterials-based immunotherapy
References
1. M. Jung et al. Advanced Materials 2022, 34, 2106516.
2. M. Kang et al. Advanced Materials 2021, 33, 2103258.
3. H. S. Sohn et al. Science Advances 2022, 8, eabo5284.
4. M. Jung et al. Advanced Materials 2022, 35, 2207719.
1. M. Jung et al. Advanced Materials 2022, 34, 2106516.
2. M. Kang et al. Advanced Materials 2021, 33, 2103258.
3. H. S. Sohn et al. Science Advances 2022, 8, eabo5284.
4. M. Jung et al. Advanced Materials 2022, 35, 2207719.


