Catalysts for CCU have been newly developed to stimulate the low intrinsic reactivity of various unused industrial by-products
The induced catalytic CO2 reaction shows direct mineral carbonation of alkaline earth metals in representative waste materials of fly ash and ferronickel slag
The catalytic mineral carbonation can contribute to Carbon Neutrality 2050 from the utilization of a large quantity of industrial by-products through the CCU technology
1 Gwanak-ro, Gwanak-gu, Seoul 08826, Republic of Korea
Tel. +82-2-880-1172/3
Copyright © College of Engineering. SNU. All rights reserved.
ISSN: 2799-5305
Professor Juhyuk Moon in the Department of Civil and Environmental Engineering has developed reaction catalysts for Carbon Capture and Utilization (CCU) application which is the critical step for achieving Carbon Neutrality 2050.
For Carbon Neutrality by 2050, many countries have set a nationally determined contribution (NDC). In the case of Korea, the 2030 NDC was raised to 40% compared to 2018 following the 2050 carbon neutrality declaration. Although Korea has been running briskly toward the target, achieving this goal is very challenging. The biggest challenge is the development of appropriate technologies for Carbon Capture, Utilization, and Storage (CCUS).
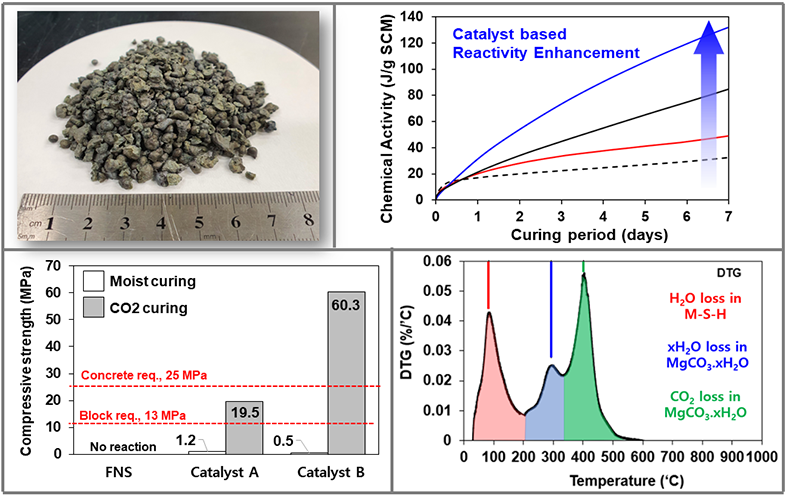
Prof. Moon proposed multiple methods for CCUS technologies. One of those is the utilization of industrial by-product produced in Korea for CCU. Ferronickel slag (FNS) is an industrial by-product from aluminum alloy production. The material is inert at ambient conditions, and very difficult to make it react with CO2 (e.g., high temperature and temperature required). Therefore, some special ingredient has to be developed to stimulate its reaction specifically with CO2 gas. The developed reaction catalyst allows the enhancement of the chemical reactivity of alkali earth metals with a minimal dosage of less than 0.1wt%. The material with enhanced reactivity can then be utilized for manufacturing “cementless” concrete or bricks.
In this case, based on the settled carbon market in Korea, carbon credits can be claimed from the direct utilization of captured CO2 gas as well as the save of cement usage for cementless concrete products. It should provide additional financial benefit along with the cost of produced materials. Prof. Moon’s research group experimentally proved that the manufactured material has enough material performance as concrete, brick, or block products under appropriate curing condition (i.e., 3 days of curing at ambient temperature and 10% of CO2 concentration)
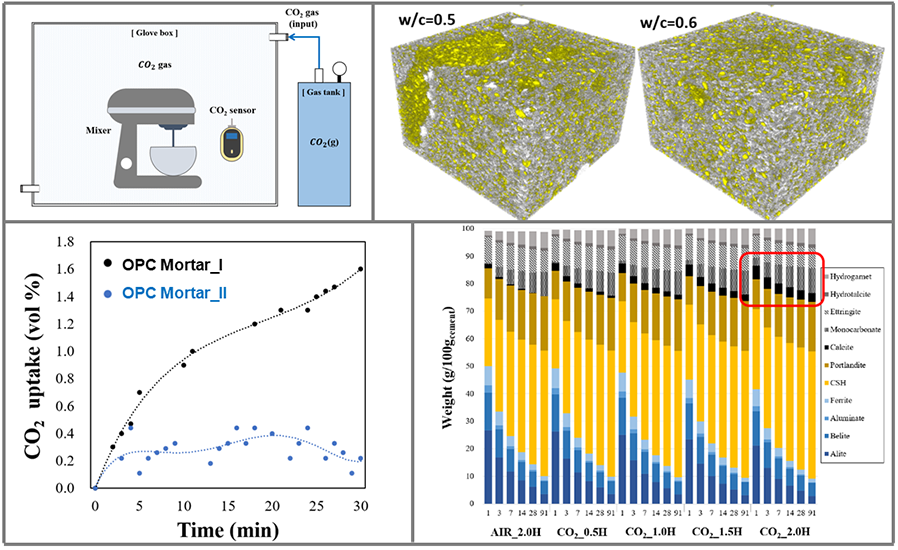
Another example of CCU technologies is the in-situ CO2 mixing for concrete. Concrete consists of cement, water, and aggregates. In cement production, a large amount of CO2 release is inevitable due to the direct decarbonation of limestone and the high temperature of kiln to produce cement materials. With around 50 million tons of cement produced every year, cement production is responsible for approximately 5-8% of CO2 emissions in Korea.
Nevertheless, concrete material itself has a huge potential as CCUS technology. In the hardened state, it contains a large volume of pores and has a favorable condition for some amount of CO2 gas to be dissolved in concrete slurry during its mixing process. If we can store 2% of CO2 gas in the concrete mixing process, around 2 million tons of CO2 can be stored in concrete every year. It is definitely a huge number compared to the storage capacities of existing CCUS technologies. Prof. Moon’s research group demonstrated that CO2 storage in concrete could be done with a short period of mixing time of less than 2 hours. The 2 hours can be further shortened because the dissolution of CO2 gas in a highly alkaline environment (i.e., initial concrete slurry state) helps for the rapid precipitation of calcite (CaCO3) and calcium monocarboaluminate hydrate (3CaO·Al2O3·CaCO3·11H2O). These two minerals are thermodynamically stable reaction products in concrete and actually help to increase long term compressive strength.
Prof. Moon noted, “CCUS technology is substantially important for achieving the NetZero by 2050. However, many CCUS technologies require additional massive solidification processes or expensive mineral carbonation steps. It is not realistic considering the CO2 storage efficiency as well as the currently tradable carbon cost. Our research group, therefore, attempts to utilize the existing industrial by-products or waste materials for CCUS. Korea is a larger emitter of CO2 gas but, at the same time, has a large amount of various waste materials available. So, we believe it can be another opportunity to contribute to the national and global carbon neutrality through the development of innovative break-through technologies. Next to the ferronickel slag, as explained, we are currently investigating the feasibility of the use of bottom ash or steel slag for the CCU technologies based on our accumulated know-how of the reaction catalyst technology for ferronickel slag and fly ash.”