Professor Sangwook Park, in the Department of Mechanical Engineering at Seoul National University, developed diverse activation methods of molybdenum disulfide (MoS2) catalyst for green hydrogen (H2) production by splitting water. Recently, the thermal oxidation mechanism of MoS2 was demonstrated, and thereby facile and controllable way to activate the MoS2 catalyst became viable.
Since current gray hydrogen production methods (e.g., steam methane reforming and coal gasification) emit lots of carbon dioxide (CO2), clean hydrogen production technologies have been actively studied to tackle energy and environmental problems and achieve carbon neutrality. Especially green hydrogen, produced by splitting water (H2O) with electricity generated by renewables (e.g., wind and solar energy), is a promising solution to produce hydrogen without CO2 emission. However, expensive and rare precious metal (e.g., Pt and Ir) based catalysts are widely used in commercial electrolysis systems due to their excellent activity and stability. Given that a massive amount of H2 is expected to be produced and utilized in diverse sectors (e.g., power generation and industry), those precious metal-based catalysts should be replaced by earth-abundant non-precious metal-based catalysts.
MoS2 has received significant attention in light of this issue because of its earth abundance and excellent active edge site for hydrogen evolution reaction (HER) in the cathode of the water-splitting system. However, its surface area mainly consists of an inactive basal plane that requires further activation from the perspective of a scale-up system. In this study, Prof. Park demonstrated the thermal oxidation (a facile and scalable activation method to generate an active edge site as a form of a pit in the basal plane of MoS2) mechanism of the inert basal plane of MoS2 by employing in situ environmental transmission electron microscopy (TEM). Although computational studies up to today estimated sulfur (S) vacancy as a thermal oxidation initiation site, this study made the first attempt to make an in situ experimental observation of the thermal oxidation of MoS2 on an atomic scale.
The results showed that the S vacancy is insufficient to initiate the thermal oxidation of the MoS2. The additional existence of adventitious carbon, an impurity on almost every synthesized catalyst surface, and its agglomerating to nanoparticles at the high oxidation temperature are need to trigger the thermal oxidation of MoS2. Density functional theory (DFT) calculation supports that the interface between the carbon nanoparticle and S vacancy site of MoS2 provides a thermodynamically favorable reaction site toward oxygen (O2) molecule dissociation into two O atoms. On top of that, the dissociated O atoms can be sequentially adsorbed at the interface for the further oxidation propagation of MoS2, leaving the pit by oxidation products (e.g., SOx and MoOx). Therefore, the thermal oxidation mechanism was understood and employed to activate the inert basal plane of the MoS2 catalyst by creating active edge sites as a form of the pit, as shown in the Figure below.
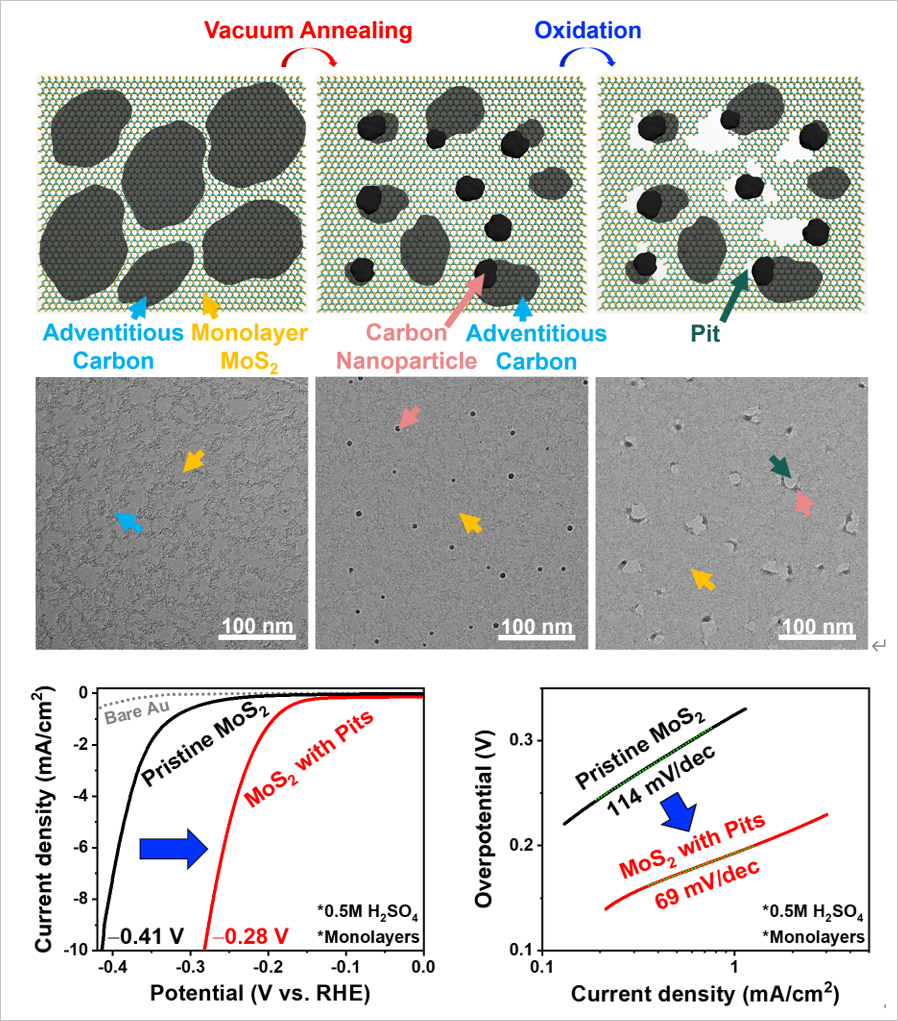
Figure Thermal oxidation mechanism and activation of MoS2 catalyst for H2 Production
Prof. Park mentioned, “this research achievement would contribute to the early realization of a future hydrogen economy by developing high-performing earth-abundant non-precious metal-based catalysts that can reduce the green H2 production price.”
The research findings were published in the internationally renowned journal, Advanced Materials, titled “Effect of Adventitious Carbon on Pit Formation of Monolayer MoS2” in 2020.
Prof. Park’s Clean Energy & Nanoheat (CLEAN) Lab actively performs research on diverse energy and environmental applications. Thermal-, energy-, and nano-engineering is employed to tackle energy and environmental challenges. 1) Clean hydrogen production and storage 2) Greenhouse gas reduction and carbon utilization 3) Water and air purification 4) Digital twin-based intelligent techno-economic analysis are studied.


