Developed multicomponent single-atom catalysts to overcome sluggish oxygen evolution reaction kinetics
Optimizing AEMWE Cell with efficient catalysts, membrane, support, and assembly to fully unleash the large-scale hydrogen production
Professor Ho Won Jang in the Department of Materials Science and Engineering has successfully developed highly active atomically dispersed electrocatalysts for oxygen evolution reaction that boosts large-scale green hydrogen production on anion exchange membrane water electrolysis.
With increasing energy demands worldwide, the consumption of fossil fuels and a huge amount of greenhouse gas generation is threatening the environment on Earth. In light of this issue, the development of sustainable energy technology has been receiving considerable attention as the solution to meeting increased energy demands and realizing carbon neutrality.
Hydrogen is currently considered a promising alternative energy carrier to replace fossil fuels due to its high energy content of 118 MJ kg-1 compared to 44 MJ kg-1 of gasoline. The eco-friendly emission of water vapor instead of greenhouse gas during hydrogen combustion is also expected to mitigate environmental issues and meet the requirements for circumventing global climate change.
However, more than 90% of hydrogen is currently produced by reforming fossil fuels, otherwise known as grey hydrogen, and a large amount of carbon dioxide is emitted during this process. Although the combustion of grey hydrogen does not evolve greenhouse gases, it is unsuitable for overall carbon neutralization.
As a sustainable approach, water electrolysis technology has been developed to produce hydrogen without emitting greenhouse gases, called green hydrogen. Electrolysis is a promising option for carbon-free hydrogen production, as it uses electricity to split water into hydrogen and oxygen. If the sources of this electricity are renewable or nuclear resources, the ultimate carbon-free energy ecosystem can be realized if the sources of this electricity are renewable or nuclear resources.
To be specific, water electrolysis takes place in a unit called an electrolyzer with two electrodes, and a water molecule is split into hydrogen and oxygen gas molecules when enough electrical voltage is applied. Unfortunately, its anodic half-reaction, oxygen evolution reaction (OER), has sluggish kinetics that significantly reduces the energy efficiency of the overall reaction. All known catalysts require high positive potentials for the onset of OER, even higher than the ideal 1.23 V, and this difference between ideal and real onset potential is defined as an overpotential. To maximize the conversion efficiency of renewable energy sources, designing superior electrocatalysts with low overpotentials is vital to achieving efficient and green hydrogen production in an electrochemical way.
Recently, the research team led by Prof. Ho Won Jang has developed a highly active electrocatalyst for OER with an ultralow overpotential below 190 mV, compared to the ~300 mV of the commercial IrO2 catalysts. The research team applied a facile electrodeposition process to synthesize layered double hydroxide support with atomically dispersed transition metal atoms. The electrocatalysts could be synthesized in less than 10 minutes, and the size of electrocatalysts was successfully scaled up to more than 20 cm2 for large-scale production.
The research team further optimized the coordination environment of the single atom catalysts (SACs) by introducing the multicomponent metal active sites, adjacent coordinative dopants, support surface engineering, and ligand adsorption to fully maximize the catalytic atomic utilization and minimize the usage of expensive platinum group metal elements.
For large-scale H2 production at a low cost, anion exchange membrane (AEM) water electrolysis has been regarded as an attractive pathway to produce high-purity hydrogen from water with less expensive non-platinum group metal (PGM) electrocatalysts and low-cost hydrocarbon-based membranes. However, the AEM electrolyzer is still in its infant stage, and the device’s performance and durability should be improved to be fully commercialized for a carbon-free hydrogen fuel ecosystem.
Recently, Prof. Jang’s research team has collaborated with Prof. Min Sang Kwon’s research team in the Department of Materials Science and Engineering and Prof. Jong Hun Kang’s research team in the Department of Chemical and Biological Engineering to develop electrocatalysts, membranes, and support materials, and successfully harmonized the key components of AEM water electrolyzer. Also, a membrane electrode assembly (MEA) was successfully prepared under a cooperation research project with the Korea Research Institute of Standards and Science (KRISS). The overall performance of the AEM water electrolyzer was evaluated and confirmed with dramatically elevated hydrogen production.
This energy-efficient water electrolysis will decrease the required working voltage to achieve a targeted green hydrogen production rate, decreasing the overall cost of green hydrogen. The cost-competitiveness of green hydrogen is eventually expected overcome grey hydrogen and other energy sources.
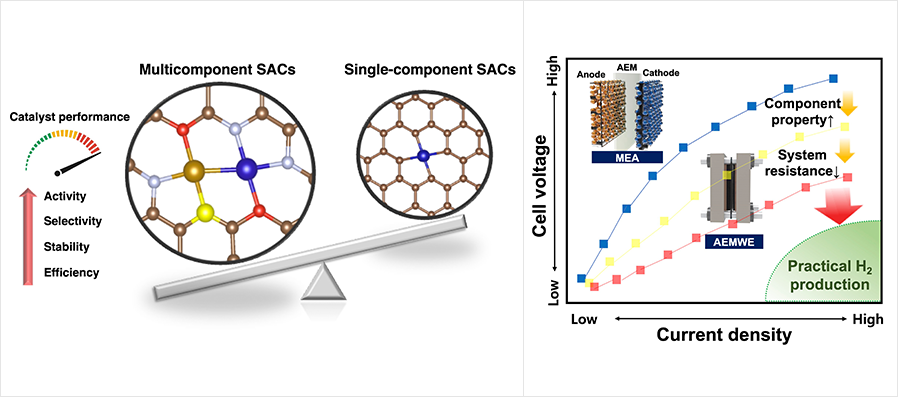
Figure
(left) Importance of multicomponent SACs and
(right) the need of developing high-performance AEMWE.


