With the development of micro-nanopore controlled separators, the implementation of organic batteries has been accelerated
The results show to be utilized in many fields such as in electric vehicles that require high energy density at low costs

From left, Professor Kisuk Kang of the SNU Department of Materials Science and Engineering,
co-first authors Songyan Bai and Byunghoon Kim
* Transition metal: Metal elements corresponding to periods 4-7, groups 3-12 in the periodic table. They are capable of easily carrying out redox.
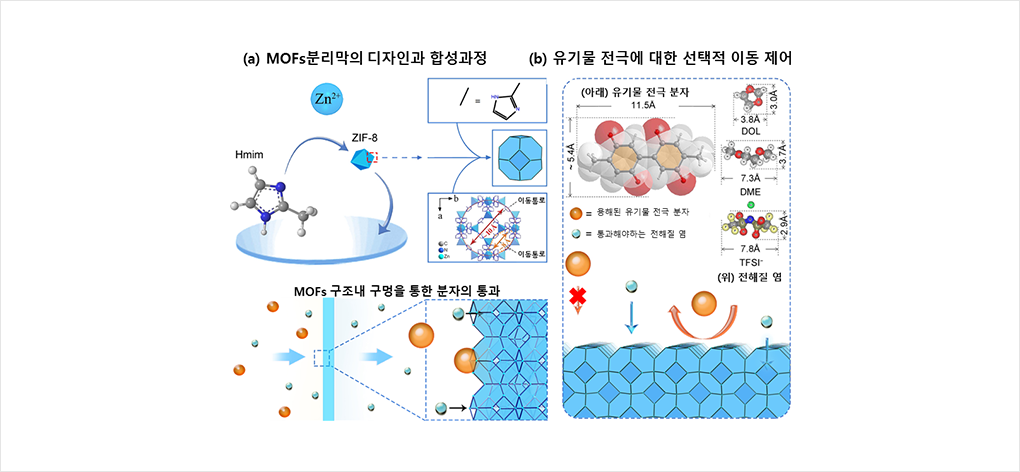
Figure 1 Selective control of organic electrode movements with MOF-gel separator
(a) A schematic showing the self-assembly of microporous MOF-gel separators (ZIF-8), and a schematic illustrating the molecular and ionic sieving process of the MOF-gel separator. A metal unit and an organic unit were combined to design and synthesize a MOF separator with nano-level movement passages.
A sol-gel process has been introduced to successfully synthesize MOFs with uniform, dense, and shock-resistant channels.
(b) The intrinsic channel size in the designed MOFs separator are smaller than those of large organic electrode molecules and larger than those corresponding to other solvents or salts. Thus, the dissolved organic electrodes can be prevented from moving to other electrodes while allowing the movement of molecules necessary for charging and discharging.
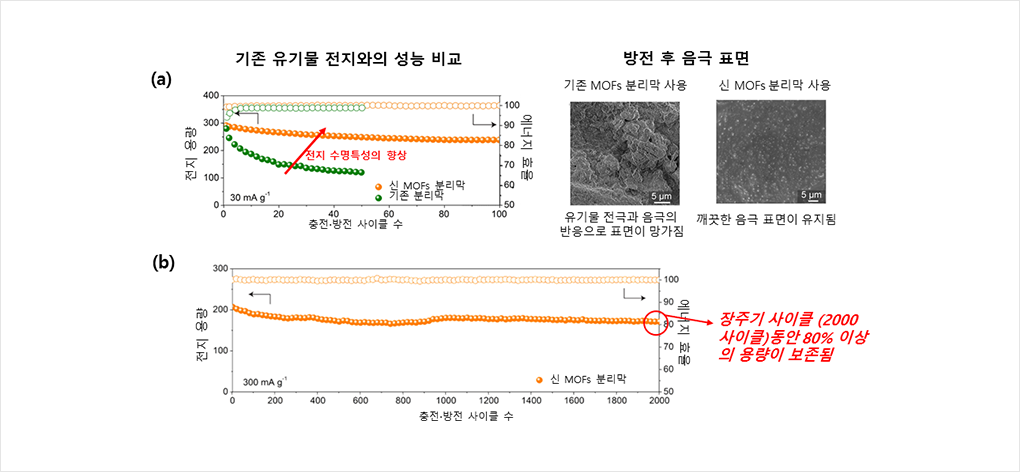
Figure 2
Dramatic improvement in organic secondary battery performance through the
introduction of MOFs separator film.
(a) (Left) The introduction of manufactured MOFs separator film into organic secondary batteries has dramatically improved the life expectancy of the battery. In the case of using pre-existing separator membranes, the battery capacity was reduced by more than half (-43.0%) within 60 cycles, while the introduction of the new MOFs separation membranes preserved ~82.0% of the battery capacity for 100 cycles.
(Right) It was found that in the case of using the existing separator, surface particles were non-uniform due to the reaction between the transferred organic electrode molecules and the cathode by observing the surface of cathodes after it is repeatedly charged and discharged. On the other hand, the introduction of MOFs separator membrane inhibited the movement of organic electrode molecules, maintaining a clean cathode surface.
(b) With the introduction of the manufactured MOFs separator, it was confirmed that most battery capacities (~82.9%) were preserved, even after long cycles of charging and discharging that took place 2000 times. Here, the capacity reduction per cycle was only 0.008%; this is an amazing result that reveals that long-term charging and discharging can be done in organic secondary batteries.


