Unveiled the cause of electrochemical degradation of oxygen-redox reaction and proposed new direction for advanced secondary battery
Research results published in the world-renowned journal <Nature Materials>

From left, Professor Kisuk Kang of the Department of Materials Science and
Engineering, Researcher Donggun Eum, Seoul National University
Professor Kisuk Kang’s research team has succeeded in identifying the degradation mechanism of the oxygen-redox reaction and has designed a new cathode material for the next-generation sodium secondary battery through this identified mechanism.
The research is expected to present a new paradigm for developing electrode materials for advanced secondary batteries with high energy density, exploiting light-weight and abundant oxygen redox capability.
As raw material prices for lithium, cobalt, and nickel continue to soar in recent years, there are difficulties in maintaining a stable supply and demand for materials for lithium secondary batteries.
Accordingly, many have conducted studies on alternative sodium secondary battery using sodium and manganese, which are abundant in reserve and have high price competitiveness, as the electrode material. However, there is an explicit limitation in the case of a sodium secondary battery: it has a lower energy density than a lithium secondary battery.
As a solution, efforts are being made worldwide to improve the performance of electrode materials through redox reactions using oxygen instead of heavy transition metals. It is remarkable that oxygen, which forms a crystal structure of a material, can additionally participate in the electrochemical reaction to increase the energy density dramatically.
However, oxygen-redox reactions are often accompanied by irreversible phenomena such as oxygen gas generation and voltage and capacity drops. Therefore, there is a great difficulty in commercialization because the material deteriorates rapidly.
Professor Kisuk Kang’s research team revealed that this irreversible characteristic was essentially caused by the bias of the oxygen stabilization mechanism during the electrochemical reaction.
By discovering three different reaction pathways in the oxygen stabilization mechanism, the team demonstrated that the σ (sigma) type stabilization mechanism which induces a strong bond between oxygen leads to the irreversibility of the oxygen-redox reaction, thus should be circumvented.
Based on this fundamental understanding, the research team presented a new design direction for a stable oxygen-redox reaction and discovered an electrode material for sodium secondary batteries with excellent energy efficiency and stable lifespan characteristics.
Regarding the research, Professor Kisuk Kang said, “It is meaningful in that it suggests a direction for designing a next-generation secondary battery material with high energy density to replace lithium secondary batteries, and it is also a research result which will contribute to the further understanding on the new class of electrode materials based on sustainable oxygen redox chemistry.”
In addition, he emphasized that “The lack of accurate understanding of the complex oxygen-redox reaction has impeded the development so far of these materials. However, this study has identified the mechanism that better explains the long-unanswered questions.”
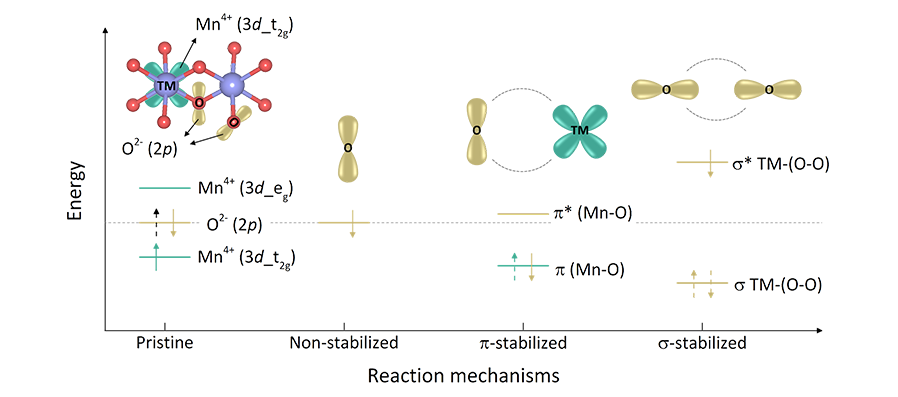
Figure
Schematic illustration of molecular orbital diagram for the three
oxygen stabilization mechanisms
The research findings were published online on March 17, in <Nature Materials>, the world’s most prestigious academic journal in the field of natural science.
For further information, please contact Prof. Kisuk Kang.


