Successful development of a Redox Battery Analysis Platform, published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS)
Attracting worldwide attention for its an effective analysis platform for complex battery systems

From left, Professor Sung Jae Kim, Professor Kisuk Kang, Ph.D. student Hyungjoo Park, Dr. Giyun Kwon of Seoul National University, and Professor Hyomin Lee of Jeju National University
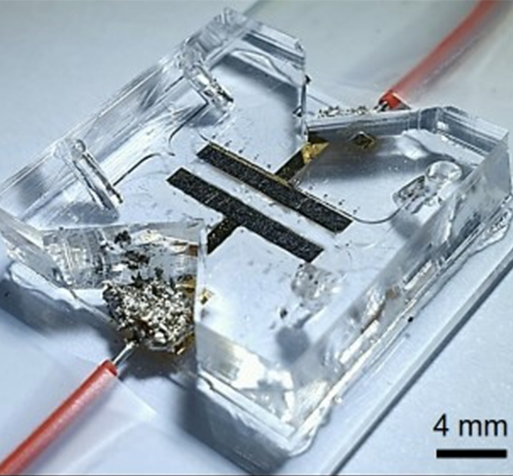
A joint research team of Professor Sung Jae Kim of the Department of Electrical and Computer Engineering and Professor Kisuk Kang of the Department of Materials Science and Engineering has succeeded in implementing a redox flow battery system capable of ion exchange between membrane-free electrolytes and has verified a platform that is capable of performing complex analysis on the system.
Instead of vanadium (a limited and toxic resource) used in current redox flow batteries, the research team used an eco-friendly organic electrolyte developed by Professor Kisuk Kang. In addition, the team combined the characteristics of the two stages of color change depending on charge states and the laminar flow in fluid mechanics, where fluids do not mix and flow side by side.
The research findings presented experimental values together with comprehensive numerical analysis and analytical solutions and received high praise for its applicability to analysis of not only redox flow battery system but also other battery systems using liquid electrolytes.
A redox flow battery is a device that converts and stores electrical and chemical energy based on the redox reaction of an anolyte and catholyte. Anolyte and catholyte within the battery are separated from each other by a physical membrane, and these membranes are used to perform ion exchange. The membrane, however, has been commented on its disadvantages due to its high cost and risk of causation of internal defects.
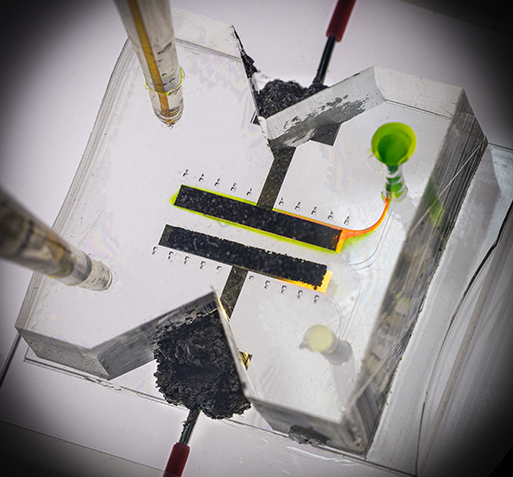
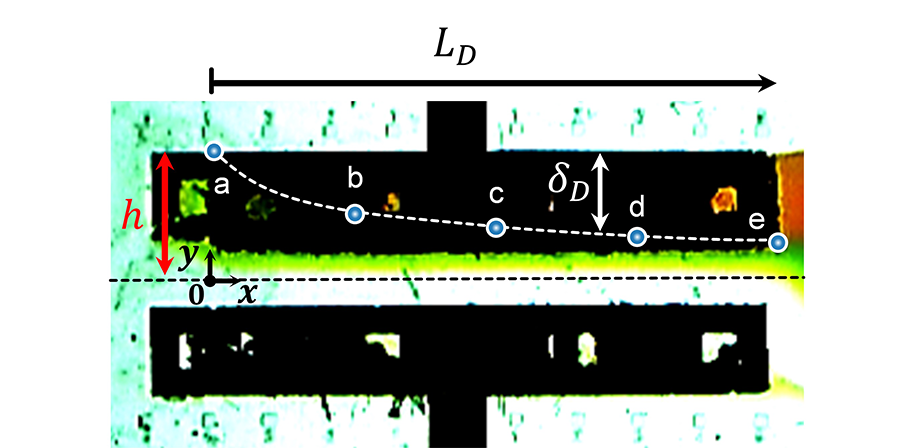
Therefore, the results of this research are meaningful in that it overcomes the shortcomings of the physical membrane of the existing commercial redox flow battery. Furthermore, the study visualizes the real-time operation of hydrodynamic and electrochemical phenomena during the analysis of a complex battery device system and presents experimental values, numerical analysis and analysis solutions in an integrated method, thus proving its value as an important analysis platform that is verified.
The team expects that they can derive the design of an efficient battery cell through the application of this study. Through the implementation of the study, ion exchange can occur even when the membrane is removed from the inside of the battery cell (Fig. 1), the degree of electrochemical reaction can be reviewed during real-time operation by visualizing the electrochemical reaction (Fig. 2), and ion-deficient layer (Fig. 3) that adversely affects battery performance can be visualized.
“This research takes full advantage of microfluidics’ most useful visualization feature as a design tool to improving battery performance,” said Professor Sung Jae Kim and Professor Kisuk Kang. “We are currently developing a platform that can be applied to other next-generation battery applications and seawater desalination devices.”
The research findings were published online on February 24, 2022, in <Proceedings of National Academy of Science (PNAS)>, the world’s leading convergence and complex academic journal.
For further information, please contact Prof. Sung Jae Kim and Prof. Kisuk Kang.