Increased the efficiency of electrochemical carbon dioxide conversion by improving mass transfer in the reactor
Expected commercialization of large-scaled conversion of electrochemical carbon dioxide in the future
From left, Ph.D. student Byungchan Jung and Professor Youngsub Lim of Seoul National University,
and Dr. Hyung-Suk Oh, and Dr. Ung Lee of Korea Institute of Science and Technology
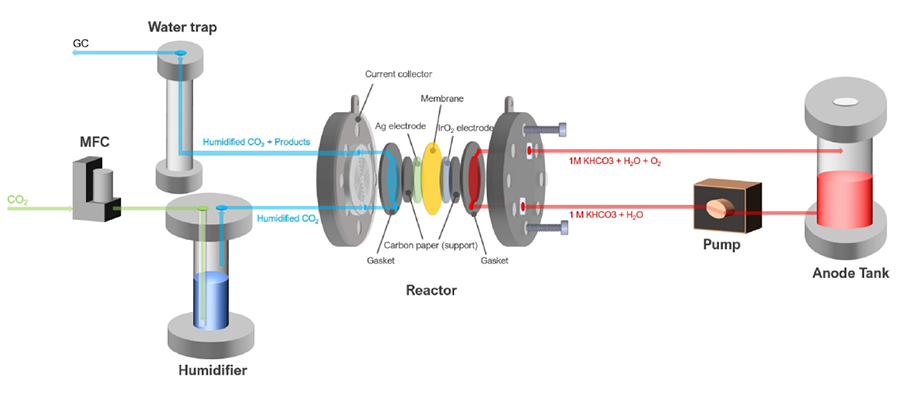
A joint research team of SNU Professor Youngsub Lim of the Department of Naval Architecture and Ocean Engineering, and KAIST Dr. Ung Lee and Hyung-Suk Oh of the Clean Energy Research Center developed a design method to increase the efficiency of carbon dioxide electrochemical conversion by improving mass transfer in the reactor.
In order to solve the recent intensifying global warming situation, we need technology to store or convert carbon dioxide, a key cause of climate change produced from the burning of fossil fuels. The electrochemical reduction of carbon dioxide has the advantage of reducing carbon dioxide and producing synthesis gas that can be used as a fuel. Nevertheless, most existing related studies are at basic level research, such as catalyst development, and there was also an insufficiency in the reactor design technology necessary for commercialization.
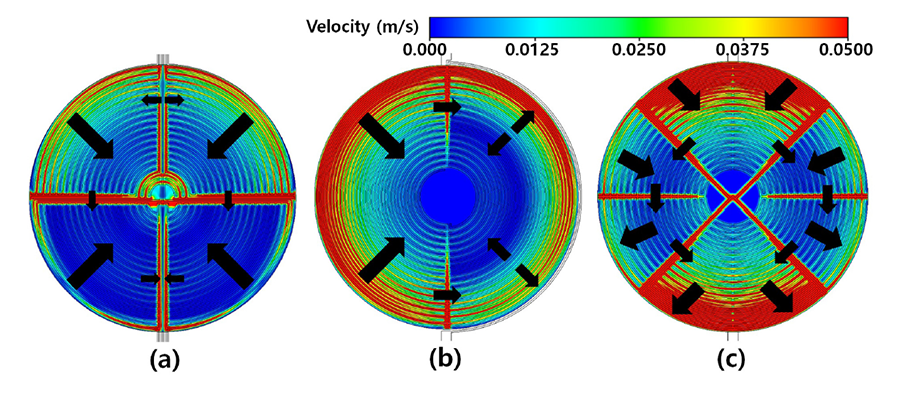
Therefore, the SNU-KIST joint research team hypothesized that external factors such as the flow of fluid in the reactor can have a dominant influence on the improvement of the reaction performance. They thus designed a structure that could maintain constant and uniform fluid flow through the computational fluid dynamics model. The team experimentally verified this structure and presented a systematic reactor design guideline.
The carbon dioxide electrochemical reduction reactor design guidelines presented in this study are expected to contribute to the future commercialization of large-scale electrochemical carbon dioxide conversion technology.
The research findings were published on November 15, 2021, in
<Chemical Engineering Journal>.
For further information, please contact Prof. Youngsub Lim.